I spent my summer working and playing in Seward,
Alaska. This post will primarily
talk about the working part, but believe me, there will be plenty dedicated to
playing as well! I volunteered as
an intern in the Eider Research Lab at the Alaska SeaLife Center for 12
weeks. Before my interview I had
to look up what an eider was, so for anyone who is wondering here is a picture.
This is a male King eider. There are four species of eiders (diving sea ducks that live
in the arctic), King eiders, Common eiders, Spectacled eiders, and Steller’s
eiders. My work related to the
Spectacled and Steller’s eiders. In
the 1970, populations of seabirds, marine mammals, and other sea life in Alaska
began declining rapidly for unknown reasons. Since the 1990s, many of those populations have stabilized,
but failed to reestablish themselves to their former abundance, and now exist
at 70-90% of previous populations.
Uncovering this mystery is one of the goals of research at the SeaLife
Center. Even if we never uncover
the mystery, the research being done has potential to help the populations of
animals in need.
If you would like more information on the Steller or
Spectacled eider projects at the SeaLife Center, you can find it here:
Working in the lab, I rarely got much hands-on animal
time. The Steller and Spectacled
eider breeding programs at the SeaLife Center have ultimate goals of
reintroduction, but currently we are in the learning phases of hatching and
rearing ducklings. Very commonly,
the eggs laid by the females are infertile, and often the ducks would die part
way through incubation. This is
where I come in!
My work primarily involved dissecting and analyzing the
eider eggs that either failed to hatch, or were not priority for hatching. This involved first acquiring metrics
of each egg, such as length, width, and weight. I documented each egg with an ID number and a picture. Then I opened the egg by cutting around
the midline (using embroidery scissors arbitrarily enough), and separating out
the yolk and albumen, much like you would if you were making an angel-food
cake. Next, if the egg were
infertile, I would try to locate and collect the germinal disc (a small, white
dot just under the surface of the yolk sac, this is where embryonic development
begins). I would then open up the
yolk and collect the perivitelline membrane (the sac surrounding the
yolk). Finally, I would mix all
the yolk components to ensure it was homogenous (with a fork – very scientific
tools at work here…) and aliquot the yolk into various vials for future
assays. For the albumen, I would
remove any shell bits I accidently created, and then mix and aliquot, much like
the yolk. Aliquots were collected
for fatty acid analysis, vitamin analysis, stable isotope analysis, and
immunoglobulin analysis, as well as an extra vial for additional assays. All of the aliquots were frozen for use
at a future time. If the egg were
fertile, my procedure varied slightly.
I would be sure to collect any vascular development present, as well as
the embryo, and then save as much clean yolk and albumen as possible. A few times, we had eggs die in the
process of hatching. Those dissections
looked into the development of the fetus, hatching position, any abnormalities
present, and the size of the yolk sac.
One of the last things the duck does before hatching is internally
absorb the remaining yolk sac. If
the yolk sac were too large, the chick would die.
During my time in the lab, I also assisted with a handful of
mussel dissections, cell and tissue culture, and virology work, and I preformed
ELISA and BCA assays on past yolk samples. ELISA (enzyme-linked immunosorbent assay) is an assay
designed to measure antigen presence in a sample. Eider chicks are born without an innate immune system, and
so any antibodies present were given straight from the mom. Therefore, by measuring the antibodies
in the yolk sample, we are able to gauge the strength of female’s immune
system. This is important for
reintroduction, as we want our chicks released to have an immune system capable
of protecting them in the wild.
Here is an example of how the assay is set up.
Once all the samples have been loaded into a 96 well plate,
along with a slew of other reagents and after a handful of incubations, the
final plate is read using a spectrophotometer, which measures the amount of
light absorbed by a substance. The
more light absorbed by a substance correlates to a higher color intensity of
each well, which in turn is correlated to higher antibody abundance.
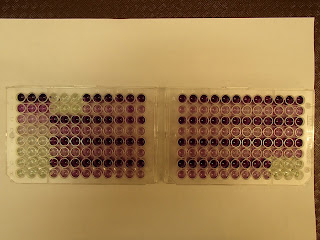
The BCA assay measures total protein concentration in the
yolk sample. It is a much simpler
assay than the ELISA, and only requires the sample and the working reagent in
each well. The final plate is read
using a spectrophotometer. The
intensity of color indicates an increased protein concentration in each
well. Here are two plates of a BCA
assay.
I really enjoyed my summer working in the Eider Research
Lab. The SeaLife Center is full of
interesting things, and I highly recommend a visit if you ever get the chance!



No comments:
Post a Comment